A simple injection to eradicate cellulite could soon be available to the public, after the announcement of a successful trial during a new study.
Endo International plc released a news statement declaring positive results last week in two identical Phase 3 RELEASE studies, in which collagenase clostridium histolyticum (CCH) was used to treat cellulite in the buttocks.
Collagenase Clostridium Histolyticum injections are currently utilised in-clinic to treat only a small number of conditions, including Peyronie’s disease; penile curvature as a result of excessive wound-healing response due to trauma/injury, and Dupuytren’s disease; a thickened linear deposit of collagen that develops in a sufferer’s hand, pulling their finger/s down towards their palm. CCH is injected to the area to break down these clusters of collagen.
“Based on our review of the Phase 3 data, we remain confident in our CCH program for cellulite—a condition that makes many women self-conscious and prompts them to seek treatment options,” said Endo’s Senior Vice President and Chief Medical Officer Matthew Davis. “If approved, CCH has the potential to be an important new treatment for cellulite and we are excited to take the next steps in that process.”
The two identical, randomised, double-blind, placebo-controlled studies involved a total of 845, 18+ year old US women (423 and 422 in each group) with moderate to severe cellulite. Each one received up to three treatments of either CCH or placebo spaced at around 21 days apart, and up to twelve injections were given during each session across the left and right buttock.
Each patient’s cellulite severity was assessed at the outset and conclusion of each treatment, both by the patient themselves and by a clinician using two photonumeric cellulite severity scales; created by Endo alongside third-party psychometric experts.
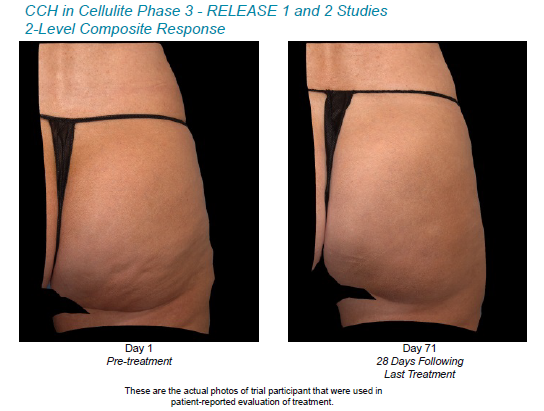
Findings showed significant improvement in the appearance of cellulite treated with CCH. 73.3% of candidates in RELEASE-1, plus 67.8% in RELEASE-2, reported “Improved,” “Very Improved” or “Very Much Improved” 71 days after treatments concluded, compared to 43.2% and 24.1% of placebo candidates respectively.
As with any injection, side effects included minor to moderate bruising or discolouration of the area.
Joely Kaufman-Janette, dermatologist at Florda’s Skin Associates and CCH clinical trial investigator, said in Endo International’s press release “the fact that CCH is a potential new injectable option to treat cellulite will be very meaningful for my patients and for the aesthetics industry overall.”
Since the treatment is still undergoing trial, it of course isn’t yet available to the public – but we imagine the industry will be keeping a very close collective eye on further developments!