The end of last week marked the official release of the TGA’s review of textured breast implants, following months of speculation, urgent meetings and updates to policy around the globe. After France, Canada and the Netherlands were moving to ban textured implants earlier this year due to increased risk of Anaplastic Large Cell Lymphoma (ALCL), the TGA called an urgent meeting in April to discuss the fate of the implants here in Australia.
The outcome of the meeting was this: all textured implant manufacturers received written requests from the TGA requesting data on the manufacturer’s implant supply here in Australia, as well as physical samples, within 10 working days. The TGA has finally reviewed responses from manufacturers, and released its official statement, review and next steps on Thursday July 11th.
“It is important to understand that the proposed cancellations and suspensions set out below are proposed.” The TGA’s announcement states. “No decision has been made to suspend or cancel the relevant products at this time.”
“Based on the laboratory and statistical review, the TGA has proposed regulatory action in relation to a number of textured implants only. Those proposed regulatory actions are either a proposal to cancel or a proposal to suspend. The TGA has also imposed new conditions on the inclusion of a number of other textured implants in the Register. The TGA has notified each of the sponsors of the relevant proposed regulatory action (or imposition of conditions, where conditions have been imposed) on 9 July 2019.”
Here are the outcomes:
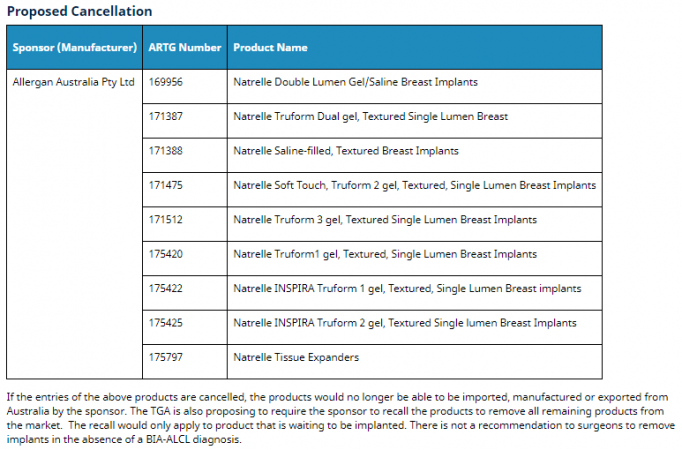
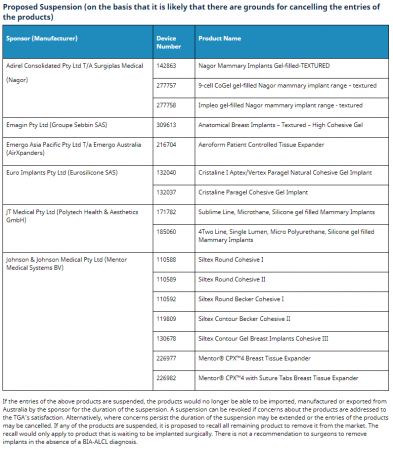
Sponsors/manufacturers have been invited to respond by July 24th.
Yesterday, the Australasian Society of Aesthetic Plastic Surgeons (ASAPS) released their official response to the TGA’s latest action.
“There has been a groundswell of media coverage about Breast Implant Illness (BII) and Breast Implant Associated-Anaplastic Large Cell Lymphoma (BIA-ALCL) leaving many who have breast implants to wonder if they are walking around with ticking time bombs in their chests,” says ASAPS’ official statement. “While media hysteria is never a good thing about any topic, there is merit in the attention on breast implants and the need to continually question what we are doing as Specialist Plastic Surgeons and whether or not patient safety can be assured.”
The statement also covers distinctions between implant types, research to date, and breast implant illness.
For more news and updates, subscribe to our weekly newsletter.